Sound Waves Scrub Your Cells: How Ultrasound Ejects Damaged Mitochondria
A new study reveals how mechanical vibrations force heart cells to package up and physically eject damaged mitochondria.
This investigation into acoustic therapy is a fascinating look at how sound waves might actively scrub our cells clean. I’ve been tracking the potential of ultrasound to reverse aspects of cellular aging for a while now—largely sparked by the research and insights Sterling Cooley has shared over the last couple of years. His work got me interested enough to experiment with a US 1000 device on my brain for potential cognitive boosts (no affiliation, just curiosity). But given that I have a recent history of cardiac challenges, seeing this technology applied specifically to heart recovery in a new paper hits home in a very practical way.
“LIPUS mechanical stimulation facilitated damaged mitochondrial excretion via migrasome-dependent mitocytosis.”
—From the research
What’s the Big Idea?
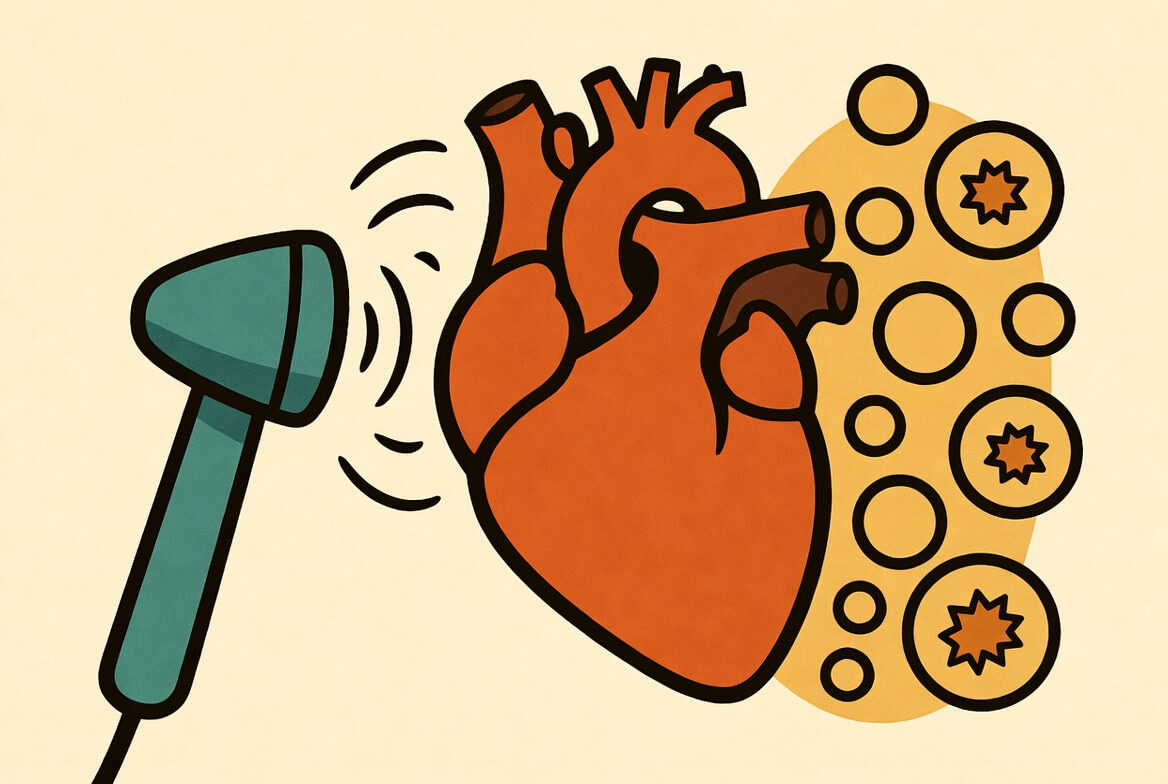
The central discovery is that low-intensity pulsed ultrasound (LIPUS) acts as a physical trigger that forces heart cells to identify, package, and vomit out their damaged energy centers. Typically, when a heart suffers a blockage and blood flow suddenly returns—a phenomenon known as ischemia-reperfusion injury—the sudden influx of oxygen wreaks havoc on mitochondria. These damaged power plants accumulate, releasing toxic signals that lead to cell death. It’s a cruel paradox where the recovery of blood flow actually causes a second wave of injury.
The analysis reveals that ultrasound waves vibrate the cellular skeleton (cytoskeleton), specifically activating a pathway involving proteins like RhoA and Myosin II. This physical shaking creates tension within the cell, which signals a “cleanup” protocol. The cell creates small vesicular sacs called migrasomes.
Think of these migrasomes as cellular trash bags. The study shows that the ultrasound stimulation forces the cell to load these bags with broken mitochondria and push them outside the cell membrane—a process called mitocytosis. By physically ejecting the garbage rather than trying to digest it internally (which can overwhelm the cell), the heart tissue recovers faster, with less scarring and better pumping function. It’s not chemical medicine; it’s mechanical hygiene.
Why It Matters and What You Can Do
This evidence constitutes a major shift in how we view tissue recovery, moving from purely chemical interventions to mechanical ones. For a long time, we’ve known mitochondria are central to longevity; when they malfunction, organs fail. This research proves that cells have an emergency ejection seat for bad mitochondria, and more importantly, that we can press that button from the outside using sound waves. Honestly, it got me inspecting my own habits—if mechanical stress on a microscopic level helps cells clean house, it reinforces why a sedentary lifestyle is so damaging. Cells seem to need physical input to maintain quality control.
While we can’t exactly replicate a controlled lab ultrasound setup at home just yet, the principles here offer some actionable directions:
Monitor the tech landscape: Ultrasound is moving from diagnostic (looking at the baby) to therapeutic (healing the tissue). Keep an eye on devices clearing FDA hurdles for “soft tissue regeneration” or “ischemic recovery,” as this field is moving fast.
Prioritize mitochondrial inputs: Since the study highlights that mitochondrial accumulation is the killer, focus on the basics that we know support mitochondrial turnover. Zone 2 cardio creates a different kind of stress that encourages mitochondrial efficiency.
Consider the “mechanical” aspect of health: The study relies on the cytoskeleton “feeling” the vibration. Activities that provide mechanical chiming—like vibration plates or even the impact of running—might offer low-level benefits in cytoskeletal signaling, though admittedly not as targeted as LIPUS.
What’s Next on the Horizon
The trajectory of this technology is pointing toward non-invasive, organ-specific detox devices. The researchers identified the specific molecular switches (YAP, Drp1, and KIF5B) that turn this cleaning process on. This suggests that in the near future, we might see therapies that combine ultrasound with specific drugs to hyper-charge this trash-removal process after a heart attack or stroke.
It opens up a wild possibility: if we can prompt heart cells to eject their junk, can we do the same for the brain or kidneys? I’ve used ultrasound for cognition, but understanding that the mechanism might involve physically shaking out cellular debris makes me wonder if this could be a future treatment for neurodegenerative conditions where protein aggregates (like plaque) build up. The researchers confirmed this pathway works in endothelial cells too, meaning it could help heal the lining of our blood vessels system-wide.
Safety, Ethics, and Caveats
The critical limitation is the gap between a highly controlled mouse model and human application. In the study, the parameters—frequency, intensity, and timing—were dialed in with extreme precision. Ultrasound at the wrong frequency can heat tissue to the point of damage or cause cavitation (tiny bubbles bursting), which tears cells apart rather than healing them.
As someone who experiments with devices like the US 1000, I see the appeal of trying this immediately, but balance is crucial. The paper used a specific low-intensity protocol. Just because some sound is good doesn’t mean louder is better. Furthermore, the study utilized genetic knockouts to prove the mechanism; humans are genetically messy. We need to ensure that stimulating this “trash ejection” doesn’t accidentally trigger other pathways, like inflammation, in surrounding tissues.
One Last Thing
It is incredibly empowering to think that our cells have these dormant sanitation crews waiting to be woken up. Biology is often smarter than we give it credit for—sometimes it just needs a little shake to get to work.
Explore the Full Study
Low-intensity pulsed ultrasound improves myocardial ischaemia‒reperfusion injury via migrasome-mediated mitocytosis
Ping Sun, Yifei Li, Weidong Yu, et al.
DOI: 10.1002/ctm2.1749