Immortal vs. Aging Hydra Reveal a Bioelectric Blueprint of Life—and What Happens When It Fades
Scientists mapped the electrical patterns across entire living organisms for the first time, uncovering striking differences between immortal and aging hydra.
If you’re not familiar with Michael Levin’s work, do yourself a favor and look him up on YouTube immediately. He’ll change the way you look at the world.
The research we’re discussing today is a study that mapped the bioelectric patterns of whole living organisms—immortal versus aging hydra—revealing fundamental differences in how these tiny freshwater creatures maintain (or lose) their form and function over time.
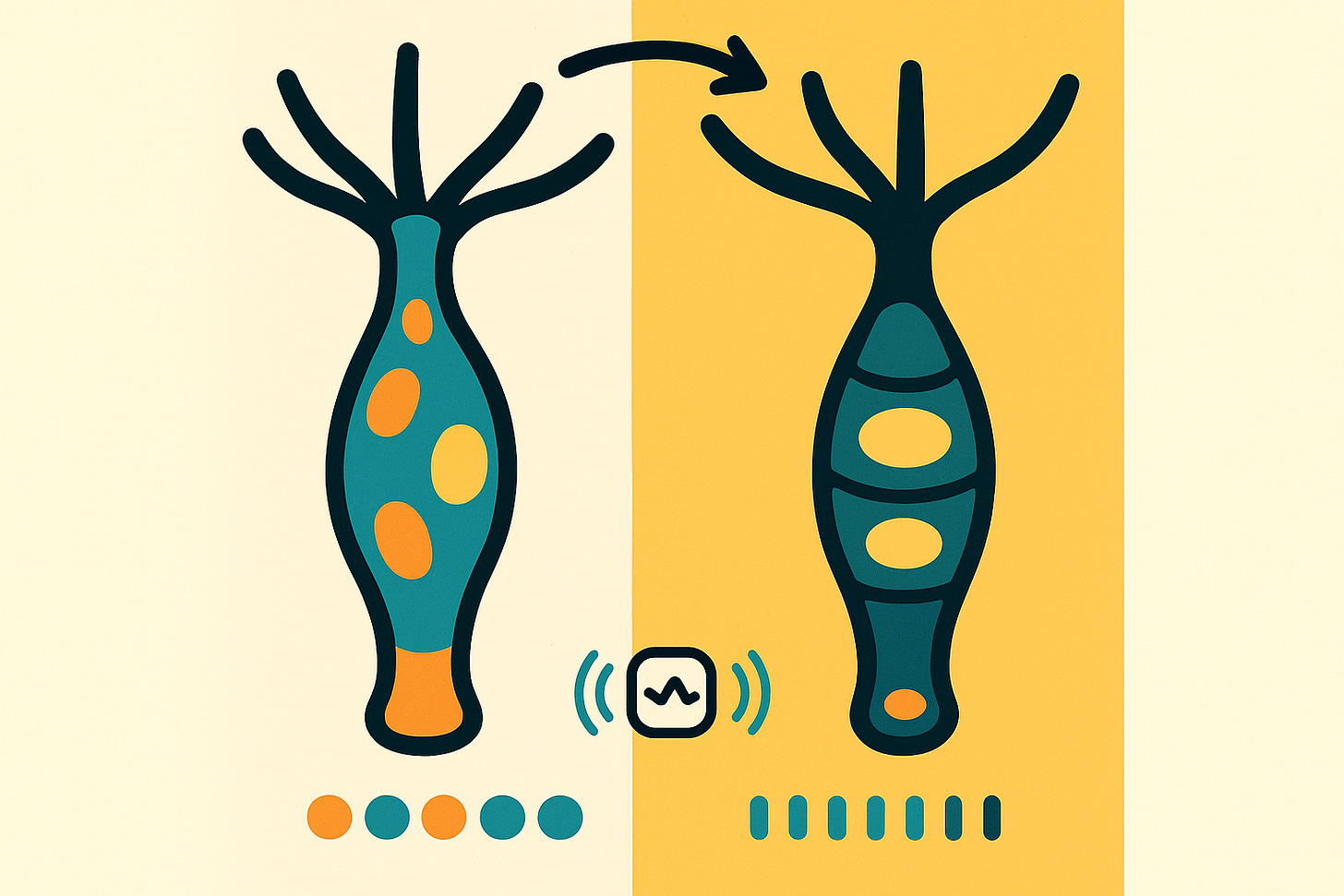
“The consensus whole-body bioelectric atlas of immortal hydra shows a consistently depolarized foot and occasionally depolarized tentacles. Immortal hydra are, on average, more depolarized and exhibit less sharply defined bioelectric patterns than old mortal hydra.”
What’s the Big Idea?
The study is a breakthrough in developmental bioelectricity, addressing a question nobody had answered before: What do the electrical patterns across an entire organism look like, and how do they change with aging?
Researchers took cold-sensitive Hydra oligactis—tiny freshwater polyps that are immortal at 22°C but age and die within 150 days at 10°C—and stained them with voltage-sensitive dyes. Using advanced fluorescence lifetime imaging microscopy (FLIM), they captured the relative membrane voltage of every single cell across the hydra’s body. Think of it as creating an electrical atlas of life itself.
Here’s what they found: Immortal hydra had consistently depolarized feet (the sticky base they use to attach to surfaces) and occasionally depolarized tentacles. Old mortal hydra? They were more hyperpolarized overall—meaning their cells held a stronger negative charge—and their bioelectric patterns were sharper, more defined. But here’s the twist: while the whole-body pattern was sharper in old hydra, the foot-to-central-body ratio became less sharp with age. The electrical signature that defines distinct body parts started to blur.
Why does this matter? Bioelectric gradients don’t just reflect what’s happening in tissues—they actively instruct cells on what to do, guiding development, regeneration, and even cancer suppression. If aging involves a degradation of these electrical instructions, that opens entirely new avenues for intervention.
Why Should You Care?
The implications are practical and profound for anyone interested in slowing aging or optimizing healthspan.
First, this is the first time anyone has created a whole-body bioelectric map of living organisms, comparing young and old individuals. We now have proof that aging isn’t just about molecular damage—it’s also about the information encoded in electrical patterns across tissues. When that information degrades, form and function degrade with it.
Second, the findings suggest testable interventions. Cells communicate through voltage gradients, and those gradients can be manipulated. Levin’s earlier work showed that forcing bioelectric patterns to become sharper in frog embryos—even when exposed to teratogens like nicotine—rescued brain development and cognitive function. Could similar approaches work for aging? The data here hint that restoring crisp bioelectric patterns might reverse some aging phenotypes.
Third, this opens the door to “electroceuticals” for longevity—drugs or devices that target ion channels to restore youthful bioelectric states. Hydra already express HCN channels (the same type that decline with age in mice), and overexpressing hcn2 has been shown to rescue defects in other organisms. If declining HCN channel function blurs bioelectric patterns in aging hydra, boosting those channels might restore their immortal state.
Finally, it’s worth noting that hydra share 80% of human aging genes. They’re not some exotic outlier—they’re a simplified, transparent model of processes that likely play out in us, too.
What’s Next on the Horizon?
The research is a starting point for a much broader program of work.
One obvious next step is testing whether bioelectric interventions—like overexpressing ion channels or applying external electric fields—can reverse aging phenotypes in hydra. If depolarization of secretory cells is necessary for foot adhesion and tentacle function, can restoring that depolarization rescue the declining abilities of old hydra to capture prey or contract?
Another question: What specific ion channels and pumps create these patterns? Mapping those would allow targeted interventions, potentially with existing drugs that modulate channel activity. Some of this work is already underway in other systems, but hydra offer a unique advantage—they’re transparent, regenerative, and you can image their entire bioelectric state in one go.
There’s also the possibility of applying similar techniques to other organisms. Humans are obviously more complex, but regional bioelectric mapping in tissues like skin, muscle, or brain could reveal aging-related changes that precede visible damage. Early detection could enable earlier intervention.
And then there’s the wild card: electromagnetic field therapies. Prior studies have shown that pulsed electromagnetic fields can extend lifespan in worms and enhance stem cell viability. If those effects work through bioelectric mechanisms, understanding the patterns revealed here could help optimize those therapies.
Safety, Ethics, and Caveats
The study is solid, but it’s early-stage work with some important limitations to keep in mind.
First, hydra aren’t humans. The findings are suggestive, not prescriptive. Just because immortal hydra are more depolarized doesn’t mean depolarizing human cells will reverse aging—it might do nothing, or worse, promote cancer (since depolarization can drive cell proliferation). Any interventions would need extensive testing in mammals before even thinking about human trials.
Second, the imaging technique itself has limitations. Cells that change their membrane voltage faster than a few hundred milliseconds (the time it takes to image a whole hydra) might not be fully captured. That’s probably fine for slow processes like development or aging, but it means the technique might miss some dynamic electrical signaling.
Third, there’s variability. Not all immortal hydra had identical patterns, and not all old hydra did either. Some of that’s biological noise, some might be functionally important. The challenge will be figuring out which differences matter and which don’t.
Finally, the dyes used (FluoVolt, VF2.1.Cl) are non-toxic in short exposures, but any intervention targeting bioelectrics would need careful safety profiling. Ion channels are everywhere in the body—messing with them indiscriminately could have unintended consequences.
What This Could Mean for You
The research is early, but it points toward actionable strategies worth considering—with appropriate caution.
First, if you’re interested in longevity, pay attention to emerging “morphoceuticals” or “electroceuticals” that target cellular voltage. These aren’t mainstream yet, but the bioelectric theory of aging is gaining traction, and drugs modulating ion channels could become part of the longevity toolkit in the next decade. Levin’s lab has already identified several FDA-approved drugs with bioelectric effects.
Second, consider that interventions like transcranial direct current stimulation (tDCS) or pulsed electromagnetic field therapy—already being explored for cognitive decline—might work through bioelectric mechanisms. The evidence is mixed, but if you’re experimenting with those approaches, this research provides a plausible mechanistic rationale.
Third, think about bioelectric health as part of your overall metabolic picture. Ion channel function depends on things like potassium, magnesium, and calcium balance—micronutrients that many people don’t optimize. While there’s no direct evidence that supplementing those will mimic the bioelectric patterns of young hydra, maintaining healthy electrolyte status is foundational for cellular function.
Finally, stay curious. Hydra regenerate, resist cancer, and—under the right conditions—live forever. Understanding how they do that, at the bioelectric level, might unlock strategies we can borrow. The fact that their aging genes overlap so much with ours suggests the lessons learned here could translate sooner than you’d think.
Explore the Full Study:
The Bioelectrics of Immortality and Mortality in Cold-Sensitive Hydra oligactis – Kapsetaki et al., 2025