Your Body's Hidden Energy Crisis: The Energy Resistance Principle (ERP) Explains Aging and Disease
The key to health isn't just about what you eat or how much you exercise—it's about how efficiently your body transforms energy.
Think about what separates a living, breathing person from a cadaver. It’s not structure—the molecules are all still there. It’s energy flow. The research we’re diving into today is a framework called the Energy Resistance Principle (ERP), and it fundamentally rethinks how we understand health, disease, and aging through the lens of energy transformation.
Published by Martin Picard and Nirosha Murugan, this isn’t just another study—it’s a unifying theory that connects mitochondrial function, inflammation, fatigue, and nearly every chronic disease you can name.
“Energy resistance (éR) is the fundamental property that enables transformation, converting the unformed energy potential of food into useful work to counteract entropy and sustain life. But excessive éR leads to dissipative loss and bioenergetic inefficiency, reductive and oxidative stress, inflammation, molecular damage, and information loss.”
What’s the Big Idea?
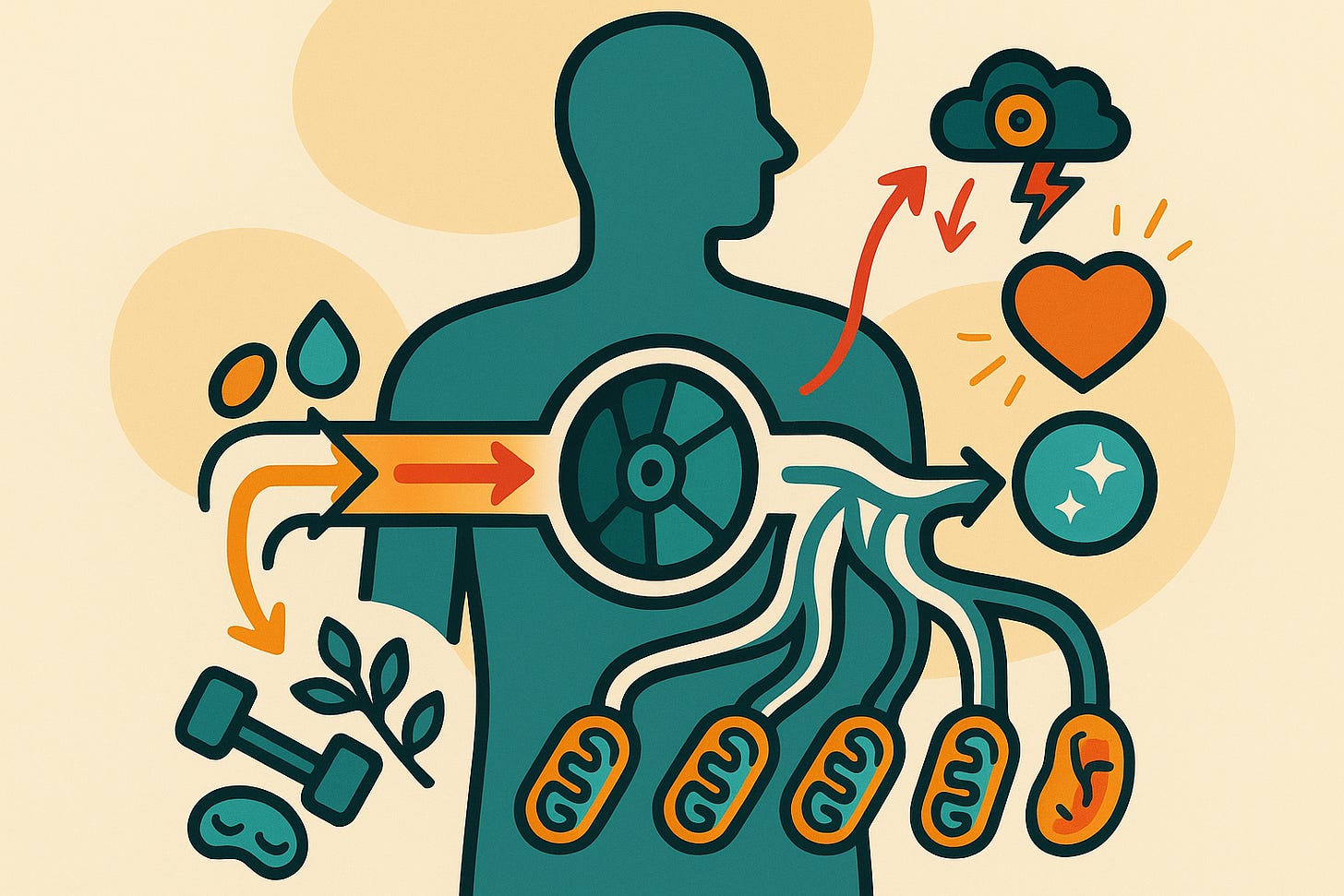
The core finding is this: your body operates like an electrical circuit, and resistance to energy flow isn’t just inevitable—it’s necessary for life. The Energy Resistance Principle shows how biology transforms raw energy from food into the work of living by precisely constraining electron flow through your mitochondria toward oxygen. Without resistance, energy would burn through you like a candle flame—instant, wasteful, destructive. With too much resistance? You get disease, inflammation, and accelerated aging.
Here’s where it gets interesting. The researchers propose that energy resistance (éR) equals your energy potential divided by your capacity for energy flow, squared. That’s éR = EP/f². Energy potential (EP) is essentially your energy demands—blood glucose levels, metabolic rate, cellular activity. Flow capacity (f²) is how much your mitochondria can actually handle, determined by things like mitochondrial number, oxygen delivery, and vascular health.
I’ll be honest—I hadn’t heard of the Energy Resistance Principle before reading this paper, but I’m loosely familiar with Martin Picard’s work since I follow him on Twitter. What I’ll say is that I’ve grown increasingly interested in mitochondrial energy as the source of the great majority of illnesses. This framework... it clicks. It explains why mitochondrial dysfunction doesn’t always show up as ATP depletion (which has puzzled researchers for years) but instead manifests as inflammation, fatigue, and metabolic chaos.
Why Should You Care?
The practical implications here are massive. This framework explains why:
Exercise works: High-intensity training temporarily maxes out your mitochondrial capacity (high éR), triggering your body to build more mitochondria and blood vessels to reduce resistance long-term. You’re literally forcing adaptive change through temporary energy stress.
Sleep heals: During sleep, your energy demands plummet (lower EP), but your flow capacity stays the same, dropping éR into the optimal zone for repair and recovery.
Inflammation signals energy trouble: When cells can’t efficiently move electrons to oxygen, they release cytokines—the molecules we call “inflammation.” The cytokine storm isn’t random; it’s your cells screaming about excessive energy resistance.
Metabolic diseases cluster together: Diabetes, heart disease, chronic fatigue—they all elevate éR through different mechanisms (high blood sugar increases EP; poor circulation decreases f²). The ERP shows why these conditions so often travel together.
Here’s something striking: people with genetic mitochondrial diseases (which directly increase éR by blocking electron flow) exhibit the same adaptations as endurance athletes—excess mitochondria, increased blood vessel growth, elevated stress hormones. Their bodies are desperately trying to compensate for constraints in the system, mimicking what happens when you push yourself hard at the gym. Except they feel it constantly, which manifests as crushing fatigue.
The researchers identify GDF15—a protein released when cells experience energy stress—as a promising biomarker for éR. High GDF15 predicts diabetes, cardiovascular disease, and even psychiatric conditions in large population studies. It’s essentially your body’s energy distress signal, and we can measure it in blood.
What’s Next on the Horizon?
The future of this framework lies in making it quantifiable and actionable. Can we develop real-time éR monitoring? Imagine a device that tracks your energy resistance the way a glucose monitor tracks blood sugar, giving you personalized feedback on when to push (exercise, fasting) and when to rest (sleep, recovery).
The researchers acknowledge they need to nail down the specifics: What exactly constitutes a “biological resistor”? How do we measure éR across different tissues and timescales? Can we target éR therapeutically—maybe through compounds that optimize mitochondrial efficiency or improve oxygen delivery?
One fascinating area for exploration: How do different interventions stack up? We know calorie restriction, NAD+ supplementation, and ketogenic diets all seem to extend healthspan. The ERP predicts they work by either decreasing EP (calorie restriction) or increasing f² (NAD+ boosting electron flow capacity). Testing these predictions could reveal which interventions work best for whom, and why.
There’s also the temperature angle. The ERP predicts that éR and temperature are intimately linked—higher resistance generates more heat as a byproduct. Recent studies show temperature matters more than metabolic rate for lifespan in mice. Who knows, maybe soon we’ll see targeted cooling therapies designed to manage energy resistance in specific tissues.
Safety, Ethics, and Caveats
The research is a theoretical framework, not a clinical intervention—yet. The authors are upfront about limitations. They’re proposing a model that needs extensive validation. Key questions remain unanswered: Can we actually measure éR accurately in living humans? Does the squared relationship (f²) hold across all biological systems, or are there exceptions? What about anaerobic organisms that don’t use oxygen—does the principle still apply?
One concern: oversimplifying complex biology. While the electrical circuit analogy is elegant, cells aren’t simple wires and resistors. They’re dynamic, adaptive, context-dependent systems. We need to be careful not to reduce everything to a single number or miss important nuances.
There’s also the risk of misapplication. If companies start marketing “éR-reducing” supplements without solid evidence, we’re back in snake oil territory. The biomarker GDF15 is promising, but it’s not specific—it rises with many stressors, not just energy resistance. Using it diagnostically requires careful interpretation within clinical context.
Finally, the framework focuses heavily on mitochondrial and metabolic processes but doesn’t fully address genetic variability, epigenetic factors, or how psychological stress translates into energy resistance (though it touches on it). The mind-mitochondria connection deserves deeper exploration.
What This Could Mean for You
The takeaway is that optimizing your health means managing energy resistance—and you have more control than you might think.
Increase your flow capacity (f²): Build more mitochondria through regular endurance exercise. Even moderate activity—brisk walking, cycling—stimulates mitochondrial biogenesis over time. Resistance training helps too by improving vascular delivery. You’re literally expanding your body’s capacity to handle energy flow.
Manage your energy potential (EP): Blood sugar spikes from processed foods, chronic stress hormones, and sleep deprivation all jack up EP without increasing flow capacity—a recipe for high éR. Consider time-restricted eating or gentle calorie moderation (not extreme restriction) to keep EP in a manageable range. Stress management through meditation, breathwork, or simple relaxation techniques can also help.
Prioritize recovery: Sleep isn’t just rest; it’s when éR drops low enough for your body to execute the repairs and adaptations that reduce long-term resistance. Aim for 7-9 hours. Create a cool, dark environment—remember, temperature and éR are linked.
Watch for warning signs: Persistent fatigue despite adequate sleep, exercise intolerance, brain fog—these could signal chronically elevated éR. If you’re experiencing them, consider asking your doctor about metabolic markers (glucose, lipids) and potentially GDF15 if it becomes clinically available.
Think long-term adaptation, not quick fixes: The ERP suggests that dramatic short-term changes (extreme fasting, overtraining) might spike éR temporarily, which isn’t necessarily bad—it’s the stimulus for adaptation. But chronic elevation is the problem. You want oscillation: challenge yourself, then recover. Rinse, repeat.
Look, this framework won’t solve everything overnight. But it offers something biomedicine has desperately needed—a simple, principled way to understand how energy, the truly fundamental currency of life, shapes health across every level of organization. That’s pretty remarkable when you think about it.
Explore the Full Study: The Energy Resistance Principle (PDF) – Picard & Murugan, 2025